Heel Pain-Plantar Fasciopathy/itis and Neurodynamics
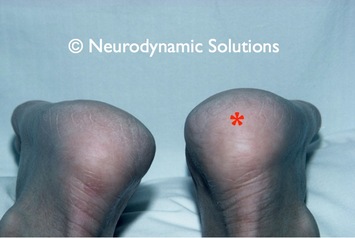
Left - Notice in the picture that the heel on the right side (patient's left) is swollen. Here is a case history of this patient in which entrapment of the tibial nerve at the ankle (tarsal tunnel syndrome) produced inflammation and pain in heel. Complete resolution of the patient's pain and swelling occurred with neurodynamic treatment.
Introduction
Heel pain is a very common and sometimes difficult problem to treat. The most popular diagnosis for pain in the heel and under the foot is plantar fasciitis. This can naturally be caused by local tissue changes but another cause is nerve entrapment, specifically in the medial and lateral plantar, tibial and medial calcaneal nerves. Nerve conduction studies have shown abnormality in nerves in patients with foot pain, providing strong evidence of neural mechanism of symptoms in some cases (Schon et al 1993). Many causes could exist for this problem, including abnormal foot biomechanics (eg. excessive pronation), anatomical variations, and neural pathologies. Magnetic resonance imaging can show Schwannomas, which are a tumour in the connective tissue of the nerve, and surgical removal has been shown to be effective in this type of case (Nawabi and Sinisi 2007). However in relation to the more physical and benign causes, there are some key facts about neurodynamic problems at the ankle that are important when considering foot pain:
Introduction
Heel pain is a very common and sometimes difficult problem to treat. The most popular diagnosis for pain in the heel and under the foot is plantar fasciitis. This can naturally be caused by local tissue changes but another cause is nerve entrapment, specifically in the medial and lateral plantar, tibial and medial calcaneal nerves. Nerve conduction studies have shown abnormality in nerves in patients with foot pain, providing strong evidence of neural mechanism of symptoms in some cases (Schon et al 1993). Many causes could exist for this problem, including abnormal foot biomechanics (eg. excessive pronation), anatomical variations, and neural pathologies. Magnetic resonance imaging can show Schwannomas, which are a tumour in the connective tissue of the nerve, and surgical removal has been shown to be effective in this type of case (Nawabi and Sinisi 2007). However in relation to the more physical and benign causes, there are some key facts about neurodynamic problems at the ankle that are important when considering foot pain:
- clearly not all foot/heel pain comes from nerves, but some does.
- nerve abnormality is an underestimated cause of foot pain. Research shows that patients with foot pain can show reduced nerve conduction in various nerves in the ankle/foot region (Schon et al 1993)).
- nerve problems can masquerade as plantar fasciitis and other local causes of heel pain.
- there should be a distinction between 'nerve entrapment' and 'neurodynamic disorders' around the ankle and foot. Nerve entrapment is frank trapping of the nerve which is mostly likely a compressive lesion (eg. tarsal tunnel syndrome). But, whilst the term 'neurodynamic disorders' includes 'nerve entrapment', the term should be used to denote other types of mechanical and physiological problems in nerve function at this location, eg. nerve irritation and inflammation due to excessive use of the ankle combined with excessive pronation. This may produce a neurodynamic disorder (probably neuritis) caused by mechanical irritation.
- tissue changes in the foot/heel eg. swelling and tenderness DO NOT EXCLUDE a neurodynamic cause. This is because the nerves can produce inflammatory changes in the tissues they innervate by a mechanism called 'neurogenic inflammation'.
 Clinical Neurodynamics, Elsevier, 2005.
Clinical Neurodynamics, Elsevier, 2005.
This article presents the key aspects related to neurodynamic diagnosis and treatment of foot pain, even when there is evidence of inflammation in the heel. The article consists of a case presentation and how the problem can be worked through in relation to clinical reasoning about the tissue mechanisms, treatment selection, performance and progression and general management. The mechanisms of neurogenic inflammation of musculoskeletal tissues are explained in more detail in Shacklock (2005, chapters 2 and 3).
Shacklock proposed a concept of neurodynamics in order to facilitate the inclusion of physiology in mechanical treatment of the nervous system (Shacklock 1995) and this is expanded on in his book Clinical Neurodynamics (Shacklock 2005).
In the context of this discussion, neurodynamics is the interaction between nervous system mechanics and physiology. In the absence of other factors, normal mechanical and physiological function will permit painfree movement and postures.
However, in the event of abnormal mechanical function, there is potential for neural structures to respond adversely, producing changes in the physiology of pain and inflammation.
What is Mechanosensitivity?
Mechanosensitivity is a gateway to neurogenic pain and is when nerve fibres convert mechanical events into impulses. This mechanism operates in normal nerves and those with pathological changes.
When normal nerve fibres are compressed or stretched experimentally, impulses are activated at the site of mechanical stress (Gray & Ritchie 1954). Body movements also evoke impulses in nerves in both normal and pathological states. In asymptomatic subjects, performance of the upper limb tension test evokes symptoms of stretch, pain, pins and needles or numbness. Importantly, the symptoms are frequently localised to the field of the median nerve (Kenneally et al 1988).
In the pathological state, a known consequence of nerve injury is increased mechanosensitivity. This is when mechanical stress applied to a damaged nerve evokes impulses (Howe et al 1976, 1977; Calvin et al 1982) and pain (Smythe & Wright 1958; Kuslich et al 1991) more easily than usual. In patients with proven peripheral neuropathy and other neurological disorders, impulses measured by microneurographic techniques have been recorded at the moment when the pathological neural structure was stressed mechanically. The median nerve and dorsal nerve roots were such structures. Particularly appealing were two observations. The nerve impulses correlated directly with the symptoms and the symptoms were elicited by placing the specific neural structure under tension (Nordin et al 1984).
KEY POINT
Nerves that are exposed to excessive or repeated mechanical force may become hypersensitive and produce impulses in both afferent and efferent directions. Afferent impulses may cause pain and efferent ones may cause inflammation in the tissues innervated by the sensitised nerve (neurogenic inflammation).
Neurogenic Inflammation
Neurogenic inflammation is inflammation that is evoked or mediated by primary afferent C-fibres. These nerve fibres are nociceptive neurones that extend from the target tissues to the spinal cord via the peripheral nerves and dorsal nerve roots. C-fibres contain neuropeptides, particularly substance P and calcitonin gene related peptide (O'Halloran & Bloom 1991; Levine et al 1993). When activated by noxious stimuli applied to the tissues, the neuropeptides are released from the C-fibres into the tissues. This efferent release of the neuropeptides is what causes neurogenic inflammation (Lembeck & Holzer 1979).
Shacklock proposed a concept of neurodynamics in order to facilitate the inclusion of physiology in mechanical treatment of the nervous system (Shacklock 1995) and this is expanded on in his book Clinical Neurodynamics (Shacklock 2005).
In the context of this discussion, neurodynamics is the interaction between nervous system mechanics and physiology. In the absence of other factors, normal mechanical and physiological function will permit painfree movement and postures.
However, in the event of abnormal mechanical function, there is potential for neural structures to respond adversely, producing changes in the physiology of pain and inflammation.
What is Mechanosensitivity?
Mechanosensitivity is a gateway to neurogenic pain and is when nerve fibres convert mechanical events into impulses. This mechanism operates in normal nerves and those with pathological changes.
When normal nerve fibres are compressed or stretched experimentally, impulses are activated at the site of mechanical stress (Gray & Ritchie 1954). Body movements also evoke impulses in nerves in both normal and pathological states. In asymptomatic subjects, performance of the upper limb tension test evokes symptoms of stretch, pain, pins and needles or numbness. Importantly, the symptoms are frequently localised to the field of the median nerve (Kenneally et al 1988).
In the pathological state, a known consequence of nerve injury is increased mechanosensitivity. This is when mechanical stress applied to a damaged nerve evokes impulses (Howe et al 1976, 1977; Calvin et al 1982) and pain (Smythe & Wright 1958; Kuslich et al 1991) more easily than usual. In patients with proven peripheral neuropathy and other neurological disorders, impulses measured by microneurographic techniques have been recorded at the moment when the pathological neural structure was stressed mechanically. The median nerve and dorsal nerve roots were such structures. Particularly appealing were two observations. The nerve impulses correlated directly with the symptoms and the symptoms were elicited by placing the specific neural structure under tension (Nordin et al 1984).
KEY POINT
Nerves that are exposed to excessive or repeated mechanical force may become hypersensitive and produce impulses in both afferent and efferent directions. Afferent impulses may cause pain and efferent ones may cause inflammation in the tissues innervated by the sensitised nerve (neurogenic inflammation).
Neurogenic Inflammation
Neurogenic inflammation is inflammation that is evoked or mediated by primary afferent C-fibres. These nerve fibres are nociceptive neurones that extend from the target tissues to the spinal cord via the peripheral nerves and dorsal nerve roots. C-fibres contain neuropeptides, particularly substance P and calcitonin gene related peptide (O'Halloran & Bloom 1991; Levine et al 1993). When activated by noxious stimuli applied to the tissues, the neuropeptides are released from the C-fibres into the tissues. This efferent release of the neuropeptides is what causes neurogenic inflammation (Lembeck & Holzer 1979).
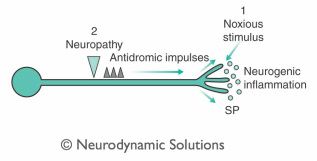
The nerve ends have generally been considered to be an important site from which inflammation is triggered (Levine et al 1985). An example of this is physical trauma. However, the idea presented here is that the C-fibre may be activated in the peripberal nerve or nerve root.
Left - Neurogenic inflammaton triggered by neuropathyThis may be the result of pathodynamics in a peripheral nerve at a tunnel or a site of mechanical irritation. In turn, impulses evoked at the lesion site may pass distally in the C-fibre toward the tissue innervated by the nerve (innervated tissue), resulting in the release of neuropeptides and subsequent inflammation in the tissues supplied by the nerve. This seems plausible in the light of evidence provided by (Bayliss 1901), who found that sectioning the dorsal nerve root and genfly tapping its distal stump resulted in neurogenic inflammation in the related dermatome. This mechanism has been confirmed more recently by Pinter and Szolcsanyi (1988) and Szolcsanyi (1988). Furthermore, impulses bound for the periphery have been found to occur in experimental nerve injury (Wall & Devor 1983) and antidromic stimulation of peripheral nerves (Chahl & Ladd 1976). Since, in the pathological state, nerves develop increased mechanosensitivity, normal body movements may trigger neurogenic inflammation.
Case History
A middle aged diabetic woman presented with a nine month history of pain and swelling in the medial and plantar aspects of her right heel. Initially, the heel pain developed gradually over several weeks, but there had been no change in her symptoms in the several months preceding the consultation. She felt pain under her heel during weight bearing as she walked. There was no history of trauma, rheumatic disease or overuse near the time of onset. This condition has been referred to as plantar fasciitis.
However, on further questioning, it became clear that this was not purely the case. In addition to the pain, symptoms of pins and needles, numbness and swelling were also present. Furthermore, the swelling was localised to the site of pain. This site corresponded to the innervation territory of the medial calcaneal nerve which is a branch of the posterior tibial nerve.
Left - Neurogenic inflammaton triggered by neuropathyThis may be the result of pathodynamics in a peripheral nerve at a tunnel or a site of mechanical irritation. In turn, impulses evoked at the lesion site may pass distally in the C-fibre toward the tissue innervated by the nerve (innervated tissue), resulting in the release of neuropeptides and subsequent inflammation in the tissues supplied by the nerve. This seems plausible in the light of evidence provided by (Bayliss 1901), who found that sectioning the dorsal nerve root and genfly tapping its distal stump resulted in neurogenic inflammation in the related dermatome. This mechanism has been confirmed more recently by Pinter and Szolcsanyi (1988) and Szolcsanyi (1988). Furthermore, impulses bound for the periphery have been found to occur in experimental nerve injury (Wall & Devor 1983) and antidromic stimulation of peripheral nerves (Chahl & Ladd 1976). Since, in the pathological state, nerves develop increased mechanosensitivity, normal body movements may trigger neurogenic inflammation.
Case History
A middle aged diabetic woman presented with a nine month history of pain and swelling in the medial and plantar aspects of her right heel. Initially, the heel pain developed gradually over several weeks, but there had been no change in her symptoms in the several months preceding the consultation. She felt pain under her heel during weight bearing as she walked. There was no history of trauma, rheumatic disease or overuse near the time of onset. This condition has been referred to as plantar fasciitis.
However, on further questioning, it became clear that this was not purely the case. In addition to the pain, symptoms of pins and needles, numbness and swelling were also present. Furthermore, the swelling was localised to the site of pain. This site corresponded to the innervation territory of the medial calcaneal nerve which is a branch of the posterior tibial nerve.
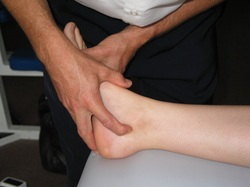
Furthermore, the nerves pass through the posterior tarsal tunnel at the medial aspect of the ankle. As well as walking, the straight leg raise (SLR) and dorsiflexion reproduced the patient's heel pain and pins and needles. Palpation of the posterior tibial nerve at the medial aspect of the ankle also elicited pain and pins and needles in the heel area (left - palpation technique for tibial nerve at the ankle).
However, on the contralateral side, these findings were absent. Palpation confirmed that there was swelling in the region of the medial part of the plantar fascia. Reduced sensation to light touch and pin prick was evident and deep pressure over the heel revealed mechanical hyperalgesia.
Analysis of Clinical Reasoning
Why would neurological symptoms coincide with pain and swelling in the heel and what could be the mechanism? Diabetic nerves are prone to entrapment and a site for this is the posterior tarsal tunnel (Mackinnon & Dellon 1988). The author suggests that the cause of the plantar fasciitis was a diabetic neuropathy of the posterior tibial nerve at the posterior tarsal tunnel. There may have been increased pressure and decreased venous return around the nerve at the tarsal tunnel. This would cause ischaemia of the nerve fibres and may result in mechanosensitivity. Furthermore, antidromic impulses in C-fibres may have been stimulated by mechanical stresses at the entrapment site, causing neurogenic inflammation in the plantar fascia. Answering the question at the beginning of this paragraph, the author believes that the neurological symptoms and inflammation coincided because the neuropathy caused the inflammation.
The logical treatment is not to stretch the nerve but, rather, move the nerve to improve venous flow and oxygenation. Mobilisation should be gentle, so that stretching the fragile nerve is avoided. However, the amplitude of movement should probably be sufficient to 'milk' the nerve, freeing it of congestion.
Treatment on the first day was as follows. IN: supine and 90º hip flexion DID: gentle knee extension so that the posterior tibial nerve slid in its tunnel and pins and needles were avoided. The mobilisations were performed rhythmically for approximately 30 seconds, after which there was a small improvement in some of the physical and subjective findings. Specifically, there was a greater range of SLR before the pins and needles appeared. However, the tenderness over the heel and the woman's ability to walk were unchanged. It seemed logical that the heel tenderness would not be influenced immediately by treatment. This is because, if the nerve entrapment were the cause of the plantar fasciitis, improvement in the neuropathy must be sustained for some time before the chain of events would reach the heel. Therefore, it was appropriate to continue to treat the nerve pathodynamics in the absence of direct evidence of an effect on the plantar fasciitis. Consequently, the same mobilisations were repeated for another 30 seconds, resulting in further improvements in SLR. At the end of the first session, there was no improvement in the patient's ability to walk. However, the response to treatment was satisfactory because the nerve physiology had improved, indicated by the improvement in range of SLR and reduction in pins and needles. If the inflammation in the heel were caused by the neuropathy, then a reduction would follow later.
The second session was four days later, when the patient reported that an easing of her pain with walking commenced two days after treatment. Since then, there had been a day by day improvement which may have indicated that the inflammation in the heel had started to resolve. Reassessment showed that the SLR range had remained stable since the first treatment, although the patient's limp, heel swelling and tenderness had reduced. The numbness was unchanged. The same treatment that was performed in the first session was repeated. Following this, there was a significant increase in range of SLR before pins and needles could be reproduced. All other physical signs remained unchanged. This time, because there had been such a sizeable improvement in SLR range, it was decided to advance the treatment further to ensure that therapeutic potency was maintained. Dorsiflexion was then performed in conjunction with knee extension, as a grade III technique (Maitland 1986). This evoked some pins and needles but these symptoms disappeared after 30 seconds of mobilisation. The technique was then repeated for another 30 seconds. After this, the SLR had improved, however, the other physical and subjective signs still remained unchanged compared with the beginning of the treatment session. An important point is that it was permissible to persist with the technique even in the absence of improvements in reassessment signs because there was already evidence that the treatment was stimulating improvements in the nerve physiology. The patient was given a home exercise that consisted of the same movements as the passive neural mobilisation. The exercises were repeated several times per day until the next appointment.
After four treatments, the patient could walk normally with no pain. There was a progressive reduction in the inflammation and tenderness around the heel and the pins and needles had been absent since the third treatment. Interestingly, the numbness did not show any signs of improvement until approximately six weeks after the final treatment.
What changes in the pathophysiology could the treatment have evoked? It is proposed that the venous flow at the nerve tunnel site improved, resulting in improved oxygenation of the nerve. This may have reduced mechanosensitivity and generation of ectopic impulses at the entrapment site. Reduction of both antidromic impulses and neurogenic inflammation in the heel may have been the final consequences.
What is the Prognosis?
Pathophysiology of the disorder offers some clues. The possibility of diabetic changes contributing to the neuropathy suggest that there may be recurrent symptoms. Furthermore, the fact that there was impaired nerve conduction (indicated by numbness) means that there was damage to the axons inside the nerve. If there had been no numbness, the prognosis may have been better. There may also be complications for the plantar fascia. Since inflammation existed for many months, long term tissue changes may have occurred. The plantar fascia may be left susceptible to pain and inflammation or even poor healing, especially if the nerve never fully recovers. In contrast, the prognosis may be improved if the diabetes is controlled optimally and the patient can regularly perform the prescribed exercises.
SUMMARY
Consistent with the definition of clinical neurodynamics (Shacklock 2005), consideration of relevant physiology in assessment and treatment of heel and foot pain is sometimes necessary. This case history of plantar fasciitis associated with peripheral neuropathy and proposed neurogenic flammation illustrates the benefits of linking mechanics with physiology of the nervous system in manual therapy.
REFERENCES
Bayliss W 1901 On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. Journal of Physiology 26:173-209
Calvin W, Devor M, Howe J 1982 Can neuralgias arise from minor demyclination? Spontaneous firing, mechanosensitivity and afterdischarge from conducting axons. Experimental Neurology 75: 755-763
Chahl L, Ladd R 1976 Local oedema and general excitation of cutaneous sensory receptors produced by electrical stimulation of the saphenous nerve in the rat. Pain 2: 25-34
Dellon A, Mackinnon S, Seiler W 1988 Susceptibility of diabetic nerve to chronic compression. Annals of Plastic Surgery 20:117-119
Gray J, Ritchie J 1954 Effects of stretch on single myclinated nerve fibres. Journal of Physiology 124: 8-99
Howe J, Calvin W, Loeser J 1976 Impulses reflected from dorsal root ganglia and from focal nerve injuries. Brain Research 116:139-144
Howe J, Loeser J, Calvin W 1977 Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain 3: 25-1
Kenneally M, Rubenach H, Elvey R 1988 The upper limb tension test: the SLR test of the arm. In: Grant E R (ed) Clinics in Physical Therapy, Physical Therapy of the Cervical and Thoracic Spine, 17. Churchill Livingstone, Edinburgh: 167-194
Kuslich S, Ulstrom C, Michael C 1991 The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthopedic Clinics of North America 22: 181-187
Lembeck F, Holzer P 1979 Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn-Schmiedeberg's Archives of Pharmacology 310:175-183
Levine J, Fields H, Basbaum A 1993 Peptides and the primary afferent nociceptor. Journal of Neuroscience 13: 2273-2286
Levine J, Moskowitz M, Basbaum A 1985 The contribution of neurogenic inflammation in experimental arthritis. Journal of Immunology 135: 843s-847s
Mackinnon S, Dellon A 1988 Surgery of the Peripheral Nerve. Thieme, New York
Maitland G D 1986 Vertebral manipulation. Butterworths, London
Nawabi D, Sinisi M 2007 Schwannoma of the posterior tibial nerve: the problem of delay in diagnosis. Journal of Bone and Joint Surgery 89B (6): 814-816
Nordin M, Nystrom B, Wallin U et al 1984 Ectopic sensory discharges and paresthesia in patients with disorders of peripheral nerves, dorsal roots and dorsal columns. Pain 20: 213-245
O'Halloran D, Bloom S 1991 Calcitonin gene related peptide: a major neuropeptide and the most powerful vasodilator known. British Medical Journal 302: 739-740
Pinter E, Szolcsanyi J 1988 Inflammatory and antunflammatory effects of antidromic stimulation of dorsal nerve roots in the rat. Agents and Actions 25: 240-242
Schon L, Glennon T, Baxter D 1993 Heel pain syndrome: electrodiagnostic support for nerve entrapment. Foot Ankle 14(3): 129-135
Shacklock M 1995 Neurodynamics. Physiotherapy 81: 9-16
Shacklock M 2005 Clinical Neurodynamics: a new system of musculoskeletal treatment. Elsevier, Oxford
Smythe M, Wright V 1958 Sciatica and the intervertebral disc. Journal of Bone and Joint Surgery 40A: 1401-1410
Szolcsanyi J 1988 Antidromic vasodilation and neurogenic inflammation. Agents and Actions 23: 4-11
Wall P, Devor M 1983 Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain 17: 321-339
However, on the contralateral side, these findings were absent. Palpation confirmed that there was swelling in the region of the medial part of the plantar fascia. Reduced sensation to light touch and pin prick was evident and deep pressure over the heel revealed mechanical hyperalgesia.
Analysis of Clinical Reasoning
Why would neurological symptoms coincide with pain and swelling in the heel and what could be the mechanism? Diabetic nerves are prone to entrapment and a site for this is the posterior tarsal tunnel (Mackinnon & Dellon 1988). The author suggests that the cause of the plantar fasciitis was a diabetic neuropathy of the posterior tibial nerve at the posterior tarsal tunnel. There may have been increased pressure and decreased venous return around the nerve at the tarsal tunnel. This would cause ischaemia of the nerve fibres and may result in mechanosensitivity. Furthermore, antidromic impulses in C-fibres may have been stimulated by mechanical stresses at the entrapment site, causing neurogenic inflammation in the plantar fascia. Answering the question at the beginning of this paragraph, the author believes that the neurological symptoms and inflammation coincided because the neuropathy caused the inflammation.
The logical treatment is not to stretch the nerve but, rather, move the nerve to improve venous flow and oxygenation. Mobilisation should be gentle, so that stretching the fragile nerve is avoided. However, the amplitude of movement should probably be sufficient to 'milk' the nerve, freeing it of congestion.
Treatment on the first day was as follows. IN: supine and 90º hip flexion DID: gentle knee extension so that the posterior tibial nerve slid in its tunnel and pins and needles were avoided. The mobilisations were performed rhythmically for approximately 30 seconds, after which there was a small improvement in some of the physical and subjective findings. Specifically, there was a greater range of SLR before the pins and needles appeared. However, the tenderness over the heel and the woman's ability to walk were unchanged. It seemed logical that the heel tenderness would not be influenced immediately by treatment. This is because, if the nerve entrapment were the cause of the plantar fasciitis, improvement in the neuropathy must be sustained for some time before the chain of events would reach the heel. Therefore, it was appropriate to continue to treat the nerve pathodynamics in the absence of direct evidence of an effect on the plantar fasciitis. Consequently, the same mobilisations were repeated for another 30 seconds, resulting in further improvements in SLR. At the end of the first session, there was no improvement in the patient's ability to walk. However, the response to treatment was satisfactory because the nerve physiology had improved, indicated by the improvement in range of SLR and reduction in pins and needles. If the inflammation in the heel were caused by the neuropathy, then a reduction would follow later.
The second session was four days later, when the patient reported that an easing of her pain with walking commenced two days after treatment. Since then, there had been a day by day improvement which may have indicated that the inflammation in the heel had started to resolve. Reassessment showed that the SLR range had remained stable since the first treatment, although the patient's limp, heel swelling and tenderness had reduced. The numbness was unchanged. The same treatment that was performed in the first session was repeated. Following this, there was a significant increase in range of SLR before pins and needles could be reproduced. All other physical signs remained unchanged. This time, because there had been such a sizeable improvement in SLR range, it was decided to advance the treatment further to ensure that therapeutic potency was maintained. Dorsiflexion was then performed in conjunction with knee extension, as a grade III technique (Maitland 1986). This evoked some pins and needles but these symptoms disappeared after 30 seconds of mobilisation. The technique was then repeated for another 30 seconds. After this, the SLR had improved, however, the other physical and subjective signs still remained unchanged compared with the beginning of the treatment session. An important point is that it was permissible to persist with the technique even in the absence of improvements in reassessment signs because there was already evidence that the treatment was stimulating improvements in the nerve physiology. The patient was given a home exercise that consisted of the same movements as the passive neural mobilisation. The exercises were repeated several times per day until the next appointment.
After four treatments, the patient could walk normally with no pain. There was a progressive reduction in the inflammation and tenderness around the heel and the pins and needles had been absent since the third treatment. Interestingly, the numbness did not show any signs of improvement until approximately six weeks after the final treatment.
What changes in the pathophysiology could the treatment have evoked? It is proposed that the venous flow at the nerve tunnel site improved, resulting in improved oxygenation of the nerve. This may have reduced mechanosensitivity and generation of ectopic impulses at the entrapment site. Reduction of both antidromic impulses and neurogenic inflammation in the heel may have been the final consequences.
What is the Prognosis?
Pathophysiology of the disorder offers some clues. The possibility of diabetic changes contributing to the neuropathy suggest that there may be recurrent symptoms. Furthermore, the fact that there was impaired nerve conduction (indicated by numbness) means that there was damage to the axons inside the nerve. If there had been no numbness, the prognosis may have been better. There may also be complications for the plantar fascia. Since inflammation existed for many months, long term tissue changes may have occurred. The plantar fascia may be left susceptible to pain and inflammation or even poor healing, especially if the nerve never fully recovers. In contrast, the prognosis may be improved if the diabetes is controlled optimally and the patient can regularly perform the prescribed exercises.
SUMMARY
Consistent with the definition of clinical neurodynamics (Shacklock 2005), consideration of relevant physiology in assessment and treatment of heel and foot pain is sometimes necessary. This case history of plantar fasciitis associated with peripheral neuropathy and proposed neurogenic flammation illustrates the benefits of linking mechanics with physiology of the nervous system in manual therapy.
REFERENCES
Bayliss W 1901 On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. Journal of Physiology 26:173-209
Calvin W, Devor M, Howe J 1982 Can neuralgias arise from minor demyclination? Spontaneous firing, mechanosensitivity and afterdischarge from conducting axons. Experimental Neurology 75: 755-763
Chahl L, Ladd R 1976 Local oedema and general excitation of cutaneous sensory receptors produced by electrical stimulation of the saphenous nerve in the rat. Pain 2: 25-34
Dellon A, Mackinnon S, Seiler W 1988 Susceptibility of diabetic nerve to chronic compression. Annals of Plastic Surgery 20:117-119
Gray J, Ritchie J 1954 Effects of stretch on single myclinated nerve fibres. Journal of Physiology 124: 8-99
Howe J, Calvin W, Loeser J 1976 Impulses reflected from dorsal root ganglia and from focal nerve injuries. Brain Research 116:139-144
Howe J, Loeser J, Calvin W 1977 Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain 3: 25-1
Kenneally M, Rubenach H, Elvey R 1988 The upper limb tension test: the SLR test of the arm. In: Grant E R (ed) Clinics in Physical Therapy, Physical Therapy of the Cervical and Thoracic Spine, 17. Churchill Livingstone, Edinburgh: 167-194
Kuslich S, Ulstrom C, Michael C 1991 The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthopedic Clinics of North America 22: 181-187
Lembeck F, Holzer P 1979 Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn-Schmiedeberg's Archives of Pharmacology 310:175-183
Levine J, Fields H, Basbaum A 1993 Peptides and the primary afferent nociceptor. Journal of Neuroscience 13: 2273-2286
Levine J, Moskowitz M, Basbaum A 1985 The contribution of neurogenic inflammation in experimental arthritis. Journal of Immunology 135: 843s-847s
Mackinnon S, Dellon A 1988 Surgery of the Peripheral Nerve. Thieme, New York
Maitland G D 1986 Vertebral manipulation. Butterworths, London
Nawabi D, Sinisi M 2007 Schwannoma of the posterior tibial nerve: the problem of delay in diagnosis. Journal of Bone and Joint Surgery 89B (6): 814-816
Nordin M, Nystrom B, Wallin U et al 1984 Ectopic sensory discharges and paresthesia in patients with disorders of peripheral nerves, dorsal roots and dorsal columns. Pain 20: 213-245
O'Halloran D, Bloom S 1991 Calcitonin gene related peptide: a major neuropeptide and the most powerful vasodilator known. British Medical Journal 302: 739-740
Pinter E, Szolcsanyi J 1988 Inflammatory and antunflammatory effects of antidromic stimulation of dorsal nerve roots in the rat. Agents and Actions 25: 240-242
Schon L, Glennon T, Baxter D 1993 Heel pain syndrome: electrodiagnostic support for nerve entrapment. Foot Ankle 14(3): 129-135
Shacklock M 1995 Neurodynamics. Physiotherapy 81: 9-16
Shacklock M 2005 Clinical Neurodynamics: a new system of musculoskeletal treatment. Elsevier, Oxford
Smythe M, Wright V 1958 Sciatica and the intervertebral disc. Journal of Bone and Joint Surgery 40A: 1401-1410
Szolcsanyi J 1988 Antidromic vasodilation and neurogenic inflammation. Agents and Actions 23: 4-11
Wall P, Devor M 1983 Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain 17: 321-339
